ZHANGJIAGANG DINSHENGLIN TRADING CO.,LTD , https://www.dslhouse.com
In the mammalian brain, the circadian clock (the main control factor) is located in the hypothalamic nucleus. However, it is not a single control factor for the daily rhythm. There are other rhythmic oscillators in the body, such as the retinal clock, which regulates many physiological activities of the nerve retina.
Zebrafish retina circadian clock  Although it is generally accepted that the retinal circadian clock responds to external light intensity to adjust visual acuity, little is known about its well-defined mechanisms and genetic effects. The retinal structure and function of zebrafish and mammals, including human approximations, are important advantages of using zebrafish as an experimental model for retinal circadian clocks. Therefore, the study of the zebrafish retinal rhythm gene may be of great significance for the understanding of the human retinal circadian clock.
Although it is generally accepted that the retinal circadian clock responds to external light intensity to adjust visual acuity, little is known about its well-defined mechanisms and genetic effects. The retinal structure and function of zebrafish and mammals, including human approximations, are important advantages of using zebrafish as an experimental model for retinal circadian clocks. Therefore, the study of the zebrafish retinal rhythm gene may be of great significance for the understanding of the human retinal circadian clock.
It is worth mentioning that the Hefei microscale material science laboratory and the researcher of the Chinese Academy of Sciences Key Laboratory of Brain Function and Disease, Huang et al. recently studied the Per 2 gene of zebrafish. The Per 2 gene is thought to play an important role in the retinal clock mechanism. This gene fragment is a light-regulating gene, and the expression of the Per 2 gene is greatly suppressed under continuous light-free conditions.
Zebrafish retina
Creating a zebrafish genetic mutant to study the retinal circadian clock <br> To study the function of a given gene fragment is usually to create a phenotype that lacks the gene to contrast with the most prevalent wild phenotypes that naturally exist. Researcher Huang and others used this method. They produced zebrafish larvae that lacked the Per 2 gene and performed various anatomical, molecular biology, and behavioral studies to compare mutant phenotypes with wild phenotypes.
Test of zebrafish's Visiobox and Zebrabox <br> One of the behavioral analyses performed by researcher Huang and others is the visual dynamic response test (OKR). Test analysis can reflect vision and contrast sensitivity. In this test, the zebrafish larvae were fixed in a petri dish with an infrared source below. The light source emits a rotating grating around the larvae. The grating causes eye movement and is captured in real time by an infrared sensitive CCD lens. Visual acuity was tested at different spatial frequencies (0.02, 0.04, 0.06, 0.08 cycles/degree). Contrast sensitivity is measured by the 'Gain' value (gain), which is the ratio of the speed of the eye movement to the speed of the stimulus stripe. A better choice for this experimental project is the use of the Visiobox product developed by Viewpoint, which is dedicated to improving the accuracy, analytical methods and reproducibility of the experiment. It will all be attributed to Visiobox's powerful automatic observation and tracking of the visual behavior of zebrafish larvae. 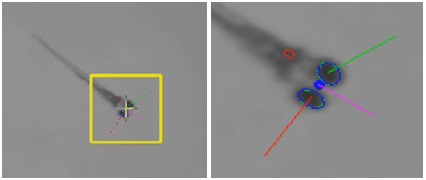
VisioBox system image output zebrafish visual response test (OKR)
The team of researcher Huang surnamed also conducted an experimental study of the visual motor response (VMR) of zebrafish larvae. The study was conducted using the ZebraBox system. ZebraBox enables automatic observation and tracking of zebrafish larvae, with up to 96 studies. This technology can be used to achieve full control of experimental conditions. Zebrafish larvae (mutant phenotype and wild phenotype) were placed in 96 well plates, respectively. Prior to the formal experiment, these larvae were placed under complete matt conditions provided by the ZebraBox system for 3.5 hours to accommodate this condition. Then suddenly adjust the lighting conditions. Every second movement of the larva is recorded by the system frame. Other tests for zebrafish larval samples include immunohistochemistry, real-time fluorescent quantitative PCR, electron microscopy imaging, etc., so that structural components and gene expression of the retina can be observed. 
Zebrafish larval behavior observation box ( ZebraBox )
Data Analysis <br> After statistical analysis of behavioral data, researchers such as Huang et al. identified individuals who lacked the per 2 gene fragment to show signs of reduced contrast sensitivity and low vision during the day. However, even the lack of the per 2 gene fragment still has an apparent dynamic response circadian rhythm.
In view of the fact that the Per 2 gene mutant showed defects in the light-on mode (photosensor operating mode) visual motor response, they decided to study the changes in retinal structure. They believed that changes in retinal structure may be the cause of vision loss. Synaptic bands of abnormal retina are often thought to cause low vision, so they conducted anatomical and molecular biology studies. Sure enough, the team of researchers in the surname Huang found that in the mutant, the synaptic band and the postsynaptic process were not connected, and even the arched dense area and the presynaptic membrane were not associated. They concluded that the per 2 gene plays an important role in the expression of cono opsin, thus leading to changes in retinal structure.
Data Conversion Conclusions <br> Based on the above, studies have shown that clock genes are important for the function of the retina to form vision. Zebrafish mutants lacking the per 2 gene showed contrast sensitivity and low vision. In particular, the zebrafish lacking the per 2 gene reduced the gain value of the visual kinetic response test during the day. However, they lack the visual kinetic response circadian rhythm even in the absence of the per 2 gene fragment. This may be because the per 2 gene fragment has little effect on the molecular clock or behavioral rhythm. In addition, the per 2 mutant exhibits abnormalities in the retinal synaptic band and a reduced number, which may be the cause of low vision.
Future Prospects of the Study <br> In summary, the zebrafish with per 2 gene mutations, although normal in the rhythm of the clock gene in the retina, are visualized due to abnormal synaptic bands. From the data point of view, the clock gene affects the visual function because it hinders nerve development and is not a circadian rhythm disorder. The study describes the main mechanism of action of the per 2 gene in regulating visual processes. It opens up new ideas for the accurate genetic mechanism research of future retinal clock control and the analogy of experience to mammals and even humans.
references
Huang D, Wang M, Yin W, Ma Y, Wang H, Xue T, Ren D and Hu B. (2018). Zebrafish Lacking Circadian Gene per2 Exhibit Visual Function Deficiency. Front. Behav. Neurosci. 12:53. 10.3389/fnbeh.2018.00053
The biological clock does not stop: revealing the activity of the retinal circadian clock by studying zebrafish
Circadian rhythm and retinal circadian clock <br> Humans and many other animal bodies, the sleep-wake cycle and other biological rhythm processes are regulated by the endogenous oscillator-biological clock. The biological clock is controlled by a series of genes whose expression is fine-tuned by the presence of light, so that the daily biological rhythm is adapted to the day and night cycle.