Compacted Oxygen Cylinder Filling System
Compacted Oxygen Cylinder Filling System
ETR oxygen cylinder filling system is consisted of the air compressor, air-water separation device, refrigerated air dryer, air dew point monitoring device, multi-stage filter, air buffer tank, air moisture monitoring device, Oxygen Generator, oxygen buffer tank, flow meter, online oxygen monitoring device, oxygen booster and cylinder filling station, smart electric control cabinet, computer monitoring system, remote network monitoring system..
For the compacted oxygen cylinder filling system, all the parts can be compacted together and skid mounted. So it is easy for the installation and operation and maintenance.
Compacted Oxygen Cylinder Filling System,Oxygen Filling Plant,Oxygen Filling Station ,Oxygen Filling Machine Hunan Eter Medical Co., Ltd. , https://www.eter-tech.com

Anti-HCV new drugs successively listed in China Interferon into the Yellow Flower
Medical Network October 12, last April, the State Food and Drug Administration's Center for Drug Evaluation (CDE) announced that a total of 10 products of 10 anti-hepatitis drugs were included in the priority review process, and the application for registration of anti-HCV drugs From then on, enter the fast lane. One year later, the hepatitis C new drug was officially opened, and new anti-viral drugs such as asurivir, darafide, simivir, sofosbuvir, and opipali were passed through the green channel. Have entered the country.
So far, there are four companies of six hepatitis C antiviral drug priority review by the Listing In China - April 24, 2017, Bristol-Myers Squibb hydrochloride Dara oseltamivir tablets and capsules Eshuruiwei The first to be approved; then on August 28, Johnson & Johnson's West Meridian capsules also passed the approval; September 21, Gilead, Aibowei's oral hepatitis C new drug sofosbuvir tablets, Obi Paoli tablets Dashabine sodium tablets were approved for CFDA marketing; in addition, Merasian's Elbasvir + Grazoprevir and Gloria's Danolive are under review.
The launch of a series of new anti-HCV drugs has opened a new chapter in the history of anti-hepatitis C virus treatment in China.
Hepatitis C new drugs at home and abroad
On December 10, 2013, the European Society of Liver Diseases published the “Guidelines for the Diagnosis and Treatment of Hepatitis C Virus (HCV) Infectionâ€. Subsequently, on April 9, 2014, the World Health Organization released the "Guide to the treatment of hepatitis C." This is a leap from the quantitative change to the qualitative change of the global hepatitis C chemotherapeutic drugs. Under the situation of new drugs, the promotion of hepatitis C treatment has entered a new era.
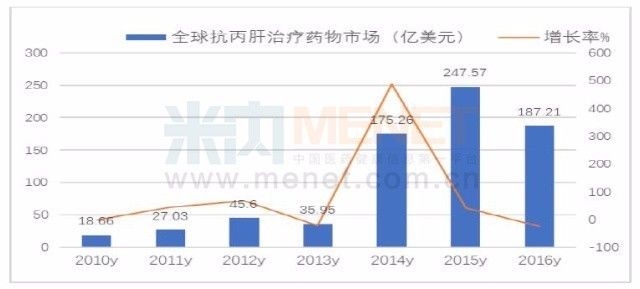
From 2011 to August 2017, the US FDA approved the listing of 10 new anti-hepatitis C virus oral drugs, which completely overturned the anti-hepatitis C treatment market. Especially after the introduction of Sofosbuvir (Soofibvir), Sofibuvir/Redipavir, Obi Pali + Paliribide + Ritonavir + Dasabide, Dalavetide + Ashuri Cocktail treatments such as Wei, Ebacevir + Gazoprovir, Sofibvir/Vipathavir, and Glecaprevir+Pibrentasvir have come out, giving hope to patients with hepatitis C and promoting the re-healing of the hepatitis C treatment market. Shuffle.
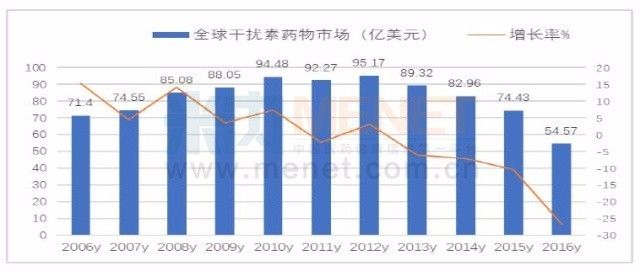
According to the financial annual report data of multinational pharmaceutical companies, the global anti-hepatitis hepatitis treatment drug market reached a peak of 24.757 billion US dollars in 2015, an increase of 41.26% over the previous year. With the competition for new anti-hepatitis C virus drugs, the market of Gilead Sovaldi in the United States has sprung up. The market changed into a normal situation; in 2016, the global anti-hepatitis treatment drug market was 18.721 billion US dollars, down 24.29% year-on-year.
Interferon status is not the same as before
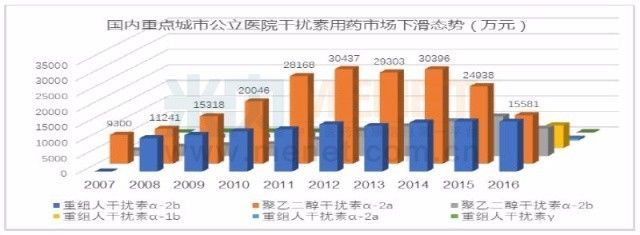
Interferon and ribavirin have been the first-line drugs for the treatment of hepatitis C. In particular, peginterferon alfa-2a and peginterferon alfa-2b have been used as engines for the anti-hepatitis C market. With the advent of anti-hepatitis C virus treatment of oral drugs, anti-hepatitis C interferon has been hit hard, and the overall market for interferon has declined. In 2016, the global interferon market fell from 9.5 billion U.S. dollars in 2012 to 5.4 billion U.S. dollars. In 2016, it dropped 26.68% from the previous year.
Oral direct-acting antiviral drugs (DAAs) are highly safe and effective, and objectively have a huge impact on interferon drugs. Interferon products will play a role in multiple sclerosis and hepatitis B treatment platforms.
According to epidemiological experts, there are currently about 10 million hepatitis C patients in China. Because hepatitis C is highly concealed, and most patients lack awareness of the disease , early detection, early diagnosis and early treatment of hepatitis C are currently difficult to achieve; patients with hepatitis C in China are already in the late stage of the disease and miss the most treatment of the disease. During the good times, it is extremely important to control hepatitis C and improve the understanding of hepatitis C. Early diagnosis and adherence to treatment.
Interferon combined with ribavirin is the main treatment option; clinically used drugs for chronic hepatitis C are mainly peginterferon alfa-2a, recombinant human interferon alpha-2b, pegylated interferon -2-2b, recombinant human interferon alpha-1b and the antiviral drug ribavirin.
Although Peg-INF/ribavirin therapy has become the gold standard for hepatitis C treatment in China, its cure rate is 44% to 70%, and in treatment, less than 2% of patients diagnosed with hepatitis C are currently based on interference. Antiviral treatment; and the later the treatment of hepatitis C, the more comorbidities, the higher the cost of treatment. Hepatitis C has brought a heavy financial burden to patients and families, and has become a serious social problem.
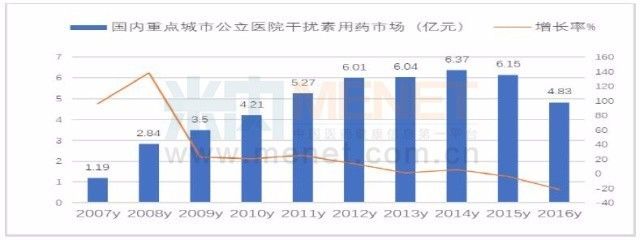
According to the data of the intranet, the sales of interferon in public hospitals in key cities in China in 2016 was 438 million yuan, a decrease of 21.46% compared with the previous year. With the introduction of new anti-hepatitis C virus drugs in China, interferon will continue to decline. With the advancement of human science, today's new drug research and development has been different from the past, we are facing the challenge of new targets; yesterday's infinite variety of scenery, tomorrow may be a smashing sand.
Domestic anti-hepatitis C-Sofibuvir development is in full swing
According to the CFDA official website data, the country has published 20 acceptance numbers for sofbvir raw materials and oral tablets, as well as two Redtipave sofosf tablets acceptance numbers. Zhengda Tianqing Pharmaceutical, Sichuan Kelun, Fujian Haixi New Drug Creation, Nanjing Xiansheng Dongyuan, Zhejiang Haizheng, Shijiazhuang Group Zhongqi, Jiangxi Shimei, Beijing Wansheng, Zhejiang Huahai and other enterprises are in full swing. The investment in research and development has exceeded 50 million yuan.
In March 2017, Shanghai Hequan Pharmaceutical Co., Ltd. was approved for clinical (CYHL1600001 Zhejiang), and Gylia (Hangzhou) Medicine Sophie Buweivirapapa Tablet (CXHL1600141 Zhejiang) was also in progress. With the launch of domestic anti-hepatitis C virus oral new drugs, chronic hepatitis C drug treatment shortcomings will be alleviated.