Bifidobacterium Bifidum,Bifidobacterium Bifidum Powder,Bifidobacterium Bifidum Probiotic,Bifidobacterium Bifidum Supplements Jiangsu Biodep Biotechnology Co. ,Ltd. , https://www.mbioda.com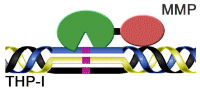
Introduction of matrix metalloproteinases and collagen nano drug delivery systems
Featured Lab: Dr. Gregg B. Field, Research Group, Torrey Pines Institute of Molecular Research , USA
Study on Matrix Metalloproteinase ( MMP ) Mechanism and Its Inhibitors
The disintegration of collagen tribodies involves a variety of diseases associated with impaired structural integrity of various components in the body (eg, arthritis and multiple sclerosis). Collagen provides a barrier to the "regionalization" of cellular activity, which is associated with tumor cell invasion and metastasis. The matrix metalloproteinase (MMP) family is thought to have collagen-dissolving activity and is a research hotspot to elucidate its mechanism of action and design inhibitors. The laboratory created and applied a "small compound library" of collagen template triple helix substrates to gain a deep understanding of the mechanism of collagen solubilization and found secondary binding sites (Exosites) associated with collagen solubilization.
Work on the activity of the triple helix polypeptide enzymes led the laboratory to consider a unique approach to designing inhibitors against collagenase. Instead of focusing on small molecule compounds, they use small protein libraries to develop highly selective drugs that act at multiple sites in the protease. As a first generation inhibitor, the triple helix transition state analog (Figure 1) has a high affinity and unique selectivity that has never been seen in the MMP field.
Figure 1. Schematic diagram of the model of the triple helix transition state analog MMP inhibitor (THP-I). Purple indicates the unhydrolyzed PO2H-CH2 bond, the MMP catalytic domain is green, and the hemoglobin binding protein-like domain is red.
Tumor cell interaction with collagen and nanotechnology-based drug delivery system (nanoDDSs)
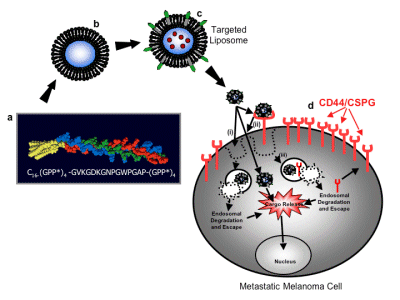
The lab's use of the micro-protein library approach to create structurally correct collagen models includes: (a) identifying specific regions within the collagen that bind to melanoma cells; and (b) identifying melanin that binds to these regions. Tumor cell receptors; (c) evaluation of these receptor-mediated intracellular and extracellular signaling results; (d) correlation of collagen three-dimensional structure to promote melanoma activity; (e) possibility of "proteolytic properties" in metastasis . As a result, a triple helix ligand of a2b1 and a3b1 integins (Integrins) and CD44/chondroitin sulfate proteoglycans was developed. These ligands were used to invent targeted nanoDDSs to directly incorporate synthetic polypeptide amphiphiles into liposome vesicles. The stable polypeptide amphiphilic nanoDDSs are selectively delivered to CD44-positive melanoma cells.
Figure 2 The basis of a targeted melanoma cell-targeted drug delivery system. a a1(IV)1263-1277 PA sequence and triple helix structure, b liposome, c drug containing a1(IV)1263-1277 PA liposome, d CD44/CSPG receptor overexpressing metastatic melanin Tumor cells. Three possible pathways for drug internalization: (i) liposome directly fused to the cell bilayer to release the drug; (ii) liposome binds to the CD44/CSPG receptor, and the liposome is fused with the cell double layer to release the drug; (iii) The liposome binds to the CD44/CSPG receptor, the receptor/liposome complex is endolyzed, and the drug is released in the inclusion body.
Studies have found that glycosylation strongly regulates melanoma cell activity, and the presence of glycosylated hydroxylysine residues in the collagen triple helix structure significantly reduces cell adhesion and spread. In summary, (a) shows for the first time that glycosylation blocks the interaction of tumor cells with the basement membrane; (b) provides a rare illustration that carbohydrates are clearly not conducive to this interaction; (c) suggests that sugars can mask cell-containing surfaces or secrete glycosides Enzymatically active "cryptographic sites" accessible to tumor cells. The lab is investigating the interaction between collagen glycosylation and tumor cell activity.