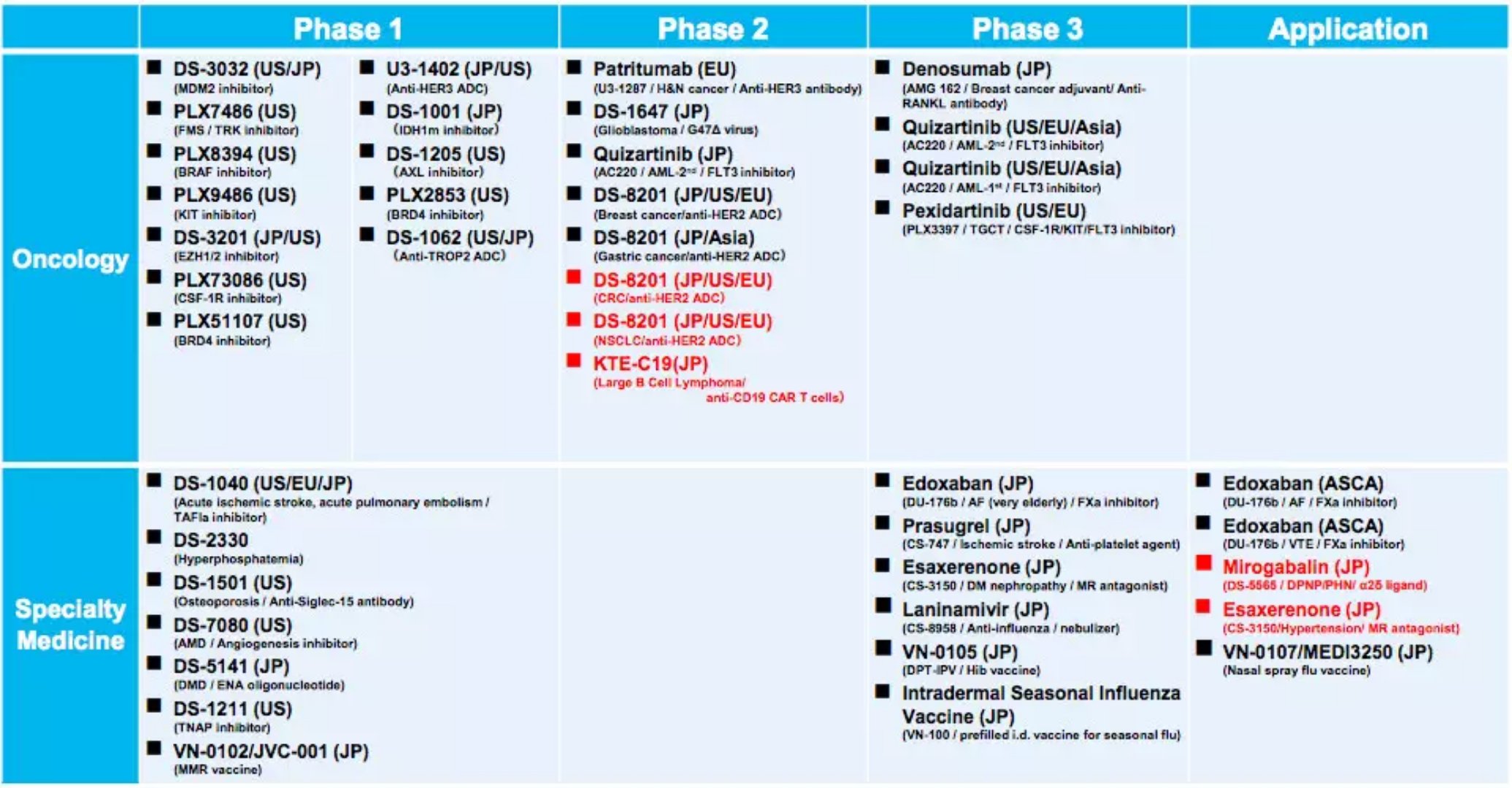
Significantly extend the lives of patients! Leukemia New Drug Program applies for global listing May 09, 2018 Source: WuXi PharmaTech Daiichi Sankyo announced today that its new drug, quizartinib, for the treatment of acute myeloid leukemia (AML) has reached its primary end point in key phase 3 clinical trials. The study showed that quizartinib monotherapy significantly prolonged the overall survival of patients with AML who had mutations in the FLT3-ITD gene compared with chemotherapy, who had cancer recurrence or resistance to therapy after first-line therapy. AML is a malignant blood and bone marrow cancer. Uncontrolled dysplastic leukocytes accumulate and accumulate in an uncontrolled manner, resulting in the formation of normal blood cells. According to a 2005-2011 survey, AML patients have a 5-year survival rate of only 26%, the lowest of all types of leukemia. Mutations in the FLT3 gene are the most common genetic variation in AML patients, while FLT3-ITD mutations are the most common mutation in the FLT3 gene, and approximately one in four AML patients carry this mutation. Patients with mutations in the FLT3-ITD gene have a worse prognosis, and their cancer recurrence is increased compared with patients who do not have this mutation, and the risk of death after relapse increases. And even with hematopoietic stem cell transplantation (HSCT), the risk of cancer recurrence after treatment will still increase. These patients urgently need effective treatment to alleviate the disease and prolong life. Quizartinib is an oral specific FLT3 inhibitor developed by the First Triad Corporation. FLT3 is a tyrosine kinase that continues to inhibit its activity leading to apoptosis in tumor cells. However, past tyrosine kinase inhibitors or FLT3 inhibitors often have a variety of side effects due to insufficient potency or specificity, which result in insufficient dose to provide sustained inhibition of FLT3 activity. Therefore, previous FLT3 inhibitors cannot treat AML as a monotherapy. Quizartinib is significantly potent compared to previous generation FLT3 inhibitors and is sufficiently specific that it completely inhibits FLT3 activity at doses tolerated by patients. Currently, it has been granted a fast-track qualification for the treatment of relapsed/refractory AML by the US FDA, and an orphan drug for the treatment of AML by the FDA and the European Medicines Agency (EMA). â–²The first three companies' research and development pipeline (Source: Daiichi Sankyo official website) In a key, open-label, global, randomized phase 3 clinical trial called QuANTUM-R, 367 AML patients with FLT3-ITD mutations received oral quizartinib monotherapy or salvage chemotherapy in a 2:1 ratio. These patients have already received standard first-line AML therapy, some of whom have received HSCT therapy, but their cancers have relapsed or become resistant to therapy. The primary endpoint of the trial was whether quizartinib could improve the overall survival (OS) of these patients compared with chemotherapy. The results showed that quizartinib reached the primary end point of this trial and that the safety and tolerability of the drug was similar to that in previous clinical trials. "In the clinical phase 3 trial of patients with relapsed/refractory AML with FLT3-ITD gene mutations, the single-agent quizartinib was the first FLT3 inhibitor to be significantly more effective than cytotoxic chemotherapy," Executive Director of the First Three Companies Dr. Antoine Yver, President and Head of Oncology R&D, said: "We would like to express our sincere gratitude to all the researchers and patients who participated in this trial. We will publish the detailed results of the QuANTUM-R clinical trial at a recent medical conference. We look forward to working with drug regulatory agencies around the world to bring quizartinib to patients as soon as possible." The First Sankyo Company plans to submit a listing application based on the QuantUM-R test results on a global scale. The company also conducts clinical phase 3 trials worldwide, testing the efficacy of quizartinib in combination with chemotherapy as a first-line therapy for patients with AML who have mutations in the FLT3-ITD gene. We expect this drug to bring effective treatment options to AML patients as soon as possible. Reference materials: [1] Daiichi Sankyo Announces Single Agent Quizartinib Significantly Prolongs Overall Survival Compared with Chemotherapy in Patients with Relapsed/Refractory AML with FLT3-ITD Mutations (QuANTUM-R Study) [2] FLT3 mutations in acute myeloid leukemia: what is the best approach in 2013? Manual Massage Collapsible Foot Spa Massager The Manual Massage Foldable Foot Spa Massager is a portable compact device designed to give your feet a relaxing massage experience. Its foldable design makes it easy to store and transport, making it ideal for the frequent traveler. Manual Massage Collapsible Foot Spa Massager,Foot Bath Massager Machine,Electric Heated Foot Spa,Foot Bath Tub Huaian Mimir Electric Appliance Co., LTD , https://www.mimirfootbath.com
Massagers typically have multiple massage rollers that apply pressure to different areas of the foot, providing a deep-tissue massage that can help relieve tension and improve circulation. Some models also feature heating and vibration to further enhance the massage experience.
To use the massager, you simply fill it with water and turn it on. The water will heat up and the massage rollers will go into action to provide a soothing massage that will help relax your feet.
Overall, the Manual Massage Foldable Foot Spa Massager is an excellent choice for anyone looking to relieve foot pain and tension in the comfort of their own home.