The Chinese Pharmacopoeia is the statutory basis for the development, production, operation, use and supervision of pharmaceuticals. The list of the 2015 edition of the Chinese Pharmacopoeia, published on June 5, 2015, contains three types of water: “purified waterâ€, “water for injection†and “sterilized water for injectionâ€. The following table shows the standard requirements for these three types of water: How do the three types of pure water prescribed by the Pharmacopoeia correspond to the pure water level in the national standard GB6682? What are the requirements of the Pharmacopoeia for TOC testing? What water should I use in drug testing? What pure water product to choose? Can ordinary water purifiers be used as water that meets the standards of the Pharmacopoeia? About Shanghai Lefeng Biotechnology Co., Ltd. Shanghai Lefeng Bio is specialized in the research, development, design and manufacture of high-end water purification and laboratory separation and purification products, and is committed to providing cutting-edge, high value-added innovative products for life sciences and biotechnology. The Lefeng product line includes laboratory pure water systems, Millipore pure water compatible consumables and laboratory separation and purification filtration products. Established ten years ago, Lefeng created its own brand RephiLe (Ruifeng), with more than 30 patents and multiple software copyrights. Products are sold to nearly 90 countries and regions around the world. For more information on RephiLe products, please visit: Le Feng official website Pay attention to RephiLe Enterprise WeChat: Le Feng pure water, pay attention to Le Feng dynamic! Le Feng article keywords: Chinese Pharmacopoeia, Munich, Shanghai, pure water, laboratory, purified water, water for injection, sterile water for injection, Munich, supervision, management, standards, analytical biochemical exhibition, Shanghai, pure water, deionized water ,DI water, distilled water, reverse osmosis water, RO water, ultrapure water, EDI, filtration, needle type, sterile, HPLC, ICP-MS, AAS, LC-MS, biochemical analyzer, sequencing, NGS high-throughput sequencing , clinical, aging machine, bottle washer, ion chromatography, filter, air filtration, carbon dioxide incubator, fermenter Waist Support Belt,Waist Back Support Belt,Waist Support Band,Medical Waist Support Belt Hebei Dingli Medical Equipment Co., Ltd. , https://www.dinglimed.com
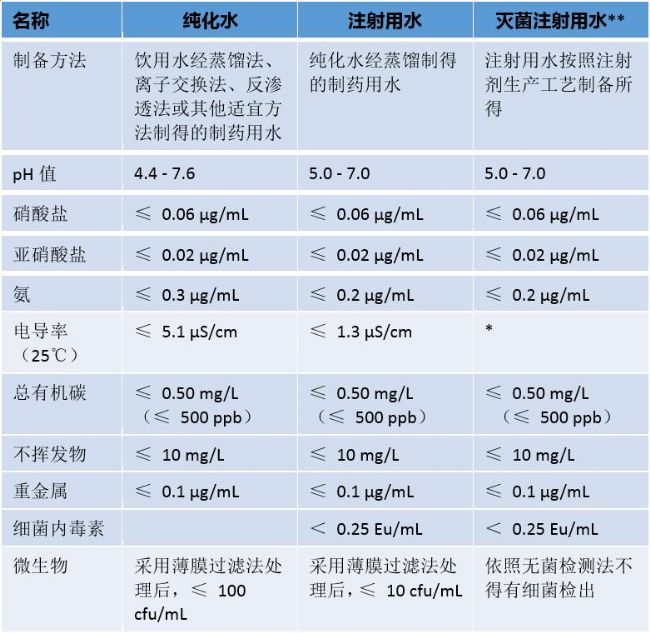
* When the conductivity is 25 °C, use an off-line conductivity meter to detect. When the marked loading is less than 10 ml, the conductivity is not more than 25 μS•cm-1; when the indicated loading is more than 10 ml, the conductivity is not more than 5 μS•cm-1.
** Sterilized water for injection requires ppb level for chloride, sulfate and calcium salt content
From the perspective of water quality:
The purified water in the 2015 Pharmacopoeia is equivalent to the third grade water of the national standard GB6682;
The water for injection in the 2015 Pharmacopoeia is close to (not as good as) the secondary water of GB GB2.
The Pharmacopoeia appendix “VIII R Total Organic Carbon Determination in Pharmaceutical Water Use†clearly states that the water system is monitored online or offline using a calibrated instrument. On-line monitoring makes it easy to measure the quality of water in real time and perform real-time process control of the water system. Off-line measurements can cause many problems, such as sampling, sampling containers, and uncontrolled environmental factors (such as vapors of organic matter). ) and other pollution.
Obviously, the method of online monitoring of total organic carbon (TOC) content in water is recommended, which is consistent with the US FDA's PAT initiative (PAT, short for Process Analytic Technology).
Although the Chinese Pharmacopoeia does not impose strict restrictions on TOC detection technology, the USP clearly stipulates that the detection method must be carried out through analytical instruments or equipment based on the principle of complete oxidation design.
Lefeng's new Genie pure water product series, the central pure water system Super-Genie series and all pure water products with TOC detection system use full oxidation method and online TOC detection technology to fully meet the requirements of pharmacopoeia and USP. 
In the test, different levels of pure water are required according to different experimental requirements:
Inductively coupled plasma mass spectrometry: use deionized water (resistivity should be no less than 18MΩ•cm), which is the high purity water required by GB33087;
Ion chromatography eluent preparation: also use deionized water (resistivity should be greater than 18MΩ•cm);
Analysis of instruments such as ICP-MS, HPLC, IC, etc.: The water requirement is extremely high, and ultrapure water should be used;
Some reagent configurations: You can use the secondary or tertiary water required by GB6682.
Lefeng's Genie G can simultaneously produce EDI secondary pure water and ultrapure water. Genie U can be configured to produce tertiary pure water and ultrapure water in a tank cycle, which is the comprehensive water demand of the complete pharmaceutical testing laboratory. The new Genie products feature innovative wireless connectivity, full traceability and comprehensive monitoring. And Genie G and Genie U can be used in one machine and two waters, which is convenient to use and saves space. 
General pure water products mainly use reverse osmosis, EDI, ion exchange and other purification technologies. Therefore, although the water quality can meet the physical and chemical indicators of water for injection and sterile water for injection, the Pharmacopoeia requires that water for injection and sterile water for injection must be used. The distillation method is prepared, so ordinary laboratory pure water equipment (without the distillation step) can not be directly used for the production of water for injection and sterile water for injection.
It should be pointed out that Lefeng's Super-Genie and Genie produced water fully complies with the physicochemical specifications and preparation methods of purified water in the Pharmacopoeia, and can be used as a production equipment for purified water in pharmaceutical factories and other related units. 