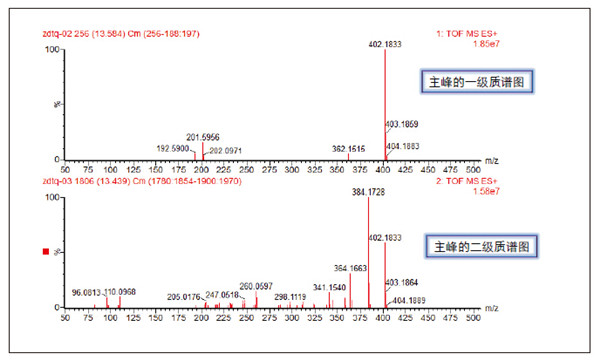
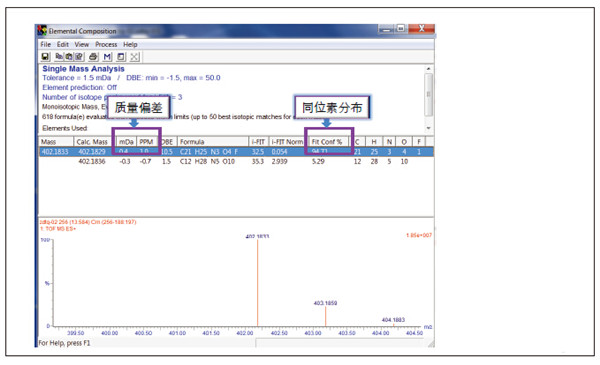
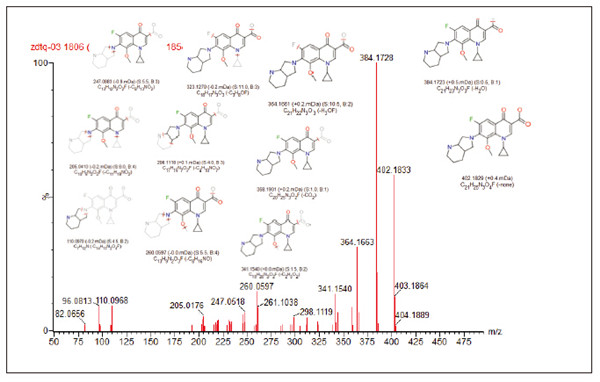
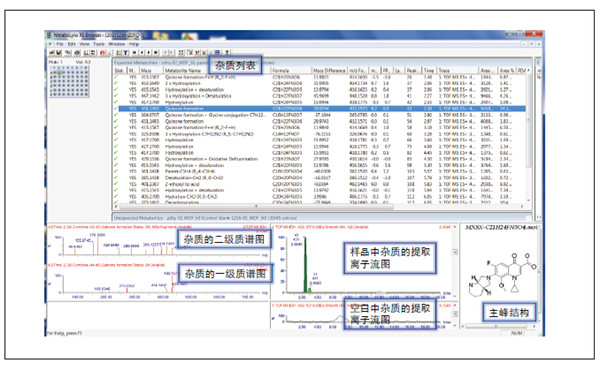
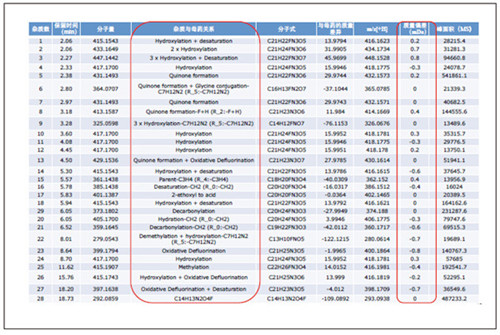
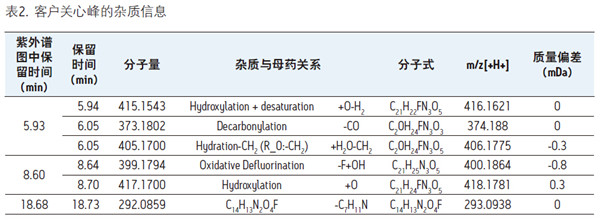
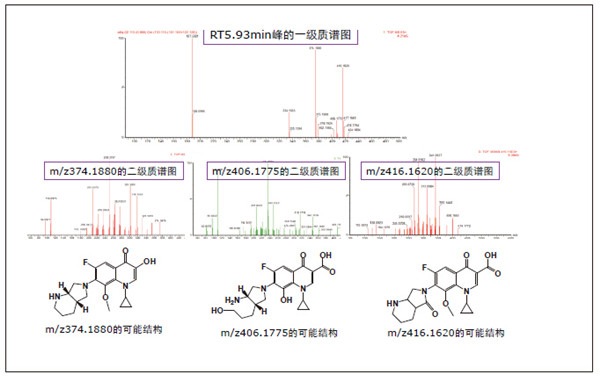
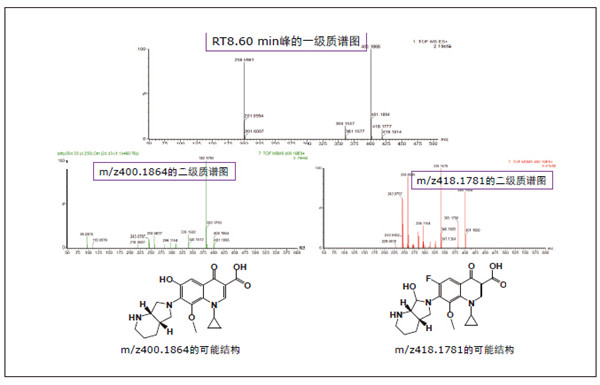
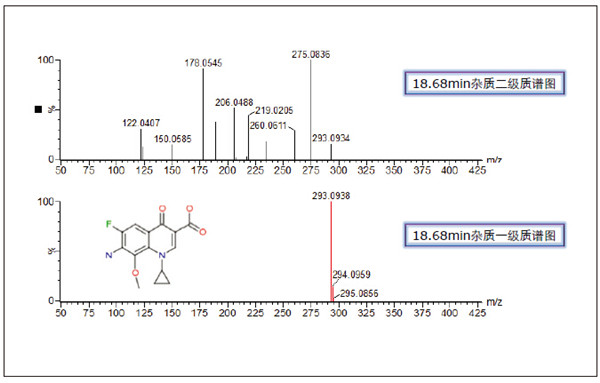
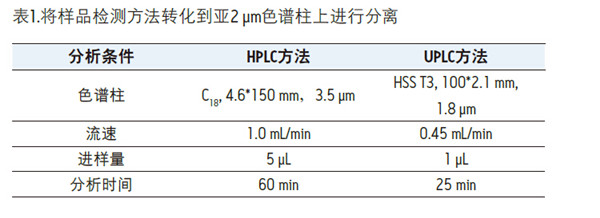
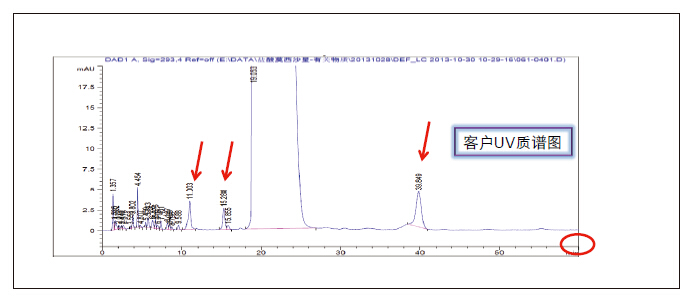
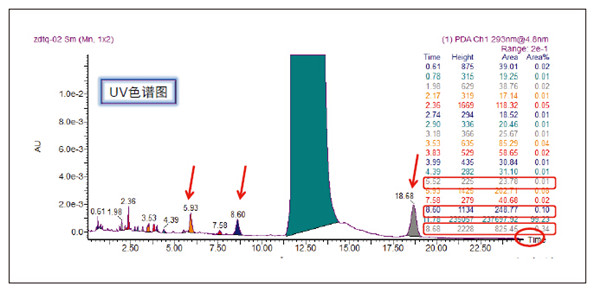
Detection and Structure Identification of Impurities in Moxifloxacin Hydrochloride Injection Wang Zhiying Waters Technology (Shanghai) Co., Ltd. Foreword: Moxifloxacin is a synthetic quinolone antibacterial and is a relatively new synthetic antibacterial. It has strong antibacterial properties, wide antibacterial spectrum, and is not easy to produce drug resistance. It is effective against common resistant bacteria, has a long half-life, and has few adverse reactions. Clinically used to treat respiratory infections, reproductive system infections, skin and soft tissue infections, etc. The original manufacturer of moxifloxacin is Bayer, Germany, and its patent protection period in China is about to expire. There are also many manufacturers in China that are carrying out imitation of moxifloxacin. However, due to the consideration of drug safety, the detection of related substances in generic drugs has attracted more attention. The Chinese Pharmacopoeia also clearly stipulates that all impurities in the drug with impurities above 0.1% need to be identified for their impurity structure (toxic drugs). The limit is even lower). Therefore, how to efficiently and quickly determine impurities in drugs has gradually become a difficult problem to be solved in the drug research process. In this paper, Waters® Ultra Performance Liquid Chromatography Time-of-Flight Mass Spectrometry (ACQUITY UPLC/Xevo G2-S QTof) was used to analyze impurities in moxifloxacin injection (light 10 days). The μm column UPLC method shortens the separation time from 60 minutes to 25 minutes, and the impurities are effectively separated. Using MassLynx and MetaboLynx software, a list of impurities containing molecular weight, molecular formula, and structure information of each impurity is automatically obtained to identify the entire impurity. The process is efficient, fast and labor intensive. Experimental conditions: experiment process: Results and discussion: Using the method converter and column selection card, the customer-supplied HPLC common liquid phase is converted into UPLC method in parallel, as shown in Table 1. Figure 3 shows the UV spectrum obtained by the customer using HPLC, and Figure 4 shows the UV spectrum obtained after conversion to the sub-2 μm column. As can be seen from the results, the analysis time was shortened from 60 minutes to 25 minutes, and the flow rate was reduced from 1.0 mL/min to 0.45 mL/min. UPLC can improve efficiency and save cost; at the same time, from the resolution and response of the spectrum See, UPLC has better resolution and higher response (injection volume is reduced from 5 μL to 1 μL). Among them, several large peaks at the red arrow in the spectrum are impurities that we pay special attention to. Figure 3. UV spectrum obtained by the customer using HPLC Figure 4. Ultraviolet spectrum obtained after conversion to a sub-2 μm column Xevo G2-S QTof mass spectrometry connected to the liquid phase UV, using MSE, a simple and practical patented technology to collect samples, can achieve sample injection once and obtain first-order mass spectrum and rich ion fragment information, including the parent ion. The results of scanning, product ion scanning, and neutral loss scanning, and loss of impurity signals are avoided. Figure 5 shows the total ion chromatogram of the first-stage mass spectrometry and the total ion chromatogram of the two-stage mass spectrometry of a sample of moxifloxacin injection (light 10 days). Figure 6 shows the primary and secondary mass spectra of the main peak. Figure 5. Moxifloxacin injection can be obtained with one injection (light 10 The molecular formula was determined by accurate mass and isotope matching using MassLynx software, and then the structure was confirmed by secondary fragment mass spectrometry. Taking the main peak as an example to illustrate the process of judging the molecular formula, as shown in Fig. 7, the molecular formula is confirmed. Secondly, the secondary fragment is automatically parsed by the software MassFragment function. As shown in Fig. 8, it can be seen that the accuracy of the fragment is also very good, and the quality deviation is small. Figure 7. Determining the molecular formula by accurate mass and isotopic distribution (taking the main peak as an example) Figure 8. Determining the molecular formula by accurate mass and isotope distribution The impurities are automatically resolved using MetaboLynx. The analysis result interface is shown in Figure 9. The impurity list can be automatically obtained, including molecular weight, molecular formula, mass deviation, peak area and structural information. The software also automatically displays the extracted ion chromatogram of each impurity in the sample, the extracted ion chromatogram in the blank, the first-order mass spectrum, and the second-order mass spectrum to make the analysis of the impurities clearer. This software provides quick access to information on all impurities on the entire sample spectrum, as shown in Figure 10. From the "mass deviation (mDa)", it can be seen that the Xevo G2-S QTof can obtain good mass accuracy with a mass deviation of 0.8 mDa. The “Impurities and Parent Drugs†column provides quick access to information about their structure. For example, "Hydroxylation" means "Structure of the main peak + O", and "Hydroxylation + desaturation" means "Structure of the main peak + O-H2". In addition, the form of "R_" such as (R_0:-CH2) refers to the structure in which the software automatically alkylates the main peak (the software has automatically given the structure), such as "Hydration-CH2 (R_0:-CH2)" That is to say, "the main peak - the structure after CH2 + H2O-CH2", its structure has also come to the fore. Figure 9. MetaboLynx's interface for automatic parsing of impurities Figure 10. List of impurities in moxifloxacin injection (light 10 days) automatically obtained by software, including impurity peak m/z, retention time, molecular weight, molecular formula, mass deviation, peak area, and specific structural information. The impurity information of 5.93 min, 8.60 min and 18.68 min in the three large peak ultraviolet spectra of particular interest to the customer is shown in Table 2. The peaks of 5.93 min and 8.60 min contain multiple impurities and are not completely separated. From Fig. 11, the first-order mass spectrum of RT 5.93 min shows that m/z 187.5981 is a double-charged molecular ion, which is the same compound as m/z 374.1880, and the content of m/z 374.1880 is higher. As can be seen from Fig. 12, 200.5981 is a double-charged molecular ion, which is the same compound as 400.1866, and the impurity concentration is high. Figures 11, 12, and 13 show the primary mass spectrum of the three peaks, the secondary mass spectrum of each impurity component, and the possible structures, respectively. Figure 11. Primary and secondary mass spectra and possible structures of impurities contained in the RT 5.93 min peak in the UV spectrum Figure 12. Primary and secondary mass spectra and possible structures of impurities contained in the RT 8.60 min peak in the UV spectrum Figure 13. Primary and secondary mass spectra and possible structures of impurities contained in the RT 18.68 min peak in the UV spectrum in conclusion: In this experiment, the conventional HPLC column detection method was parallelized into a sub-2 μm UPLC column, and the analysis time was shortened from 60 min to 25 min, which greatly improved the analysis efficiency, and the flow rate was reduced from 1 mL/min to 0.45 mL/min. Saves solvent costs. The resolution and peaking are consistent with the original spectrum. The Xevo G2-S QTof can be used to obtain the most accurate molecular weight with an impurity mass deviation of less than 0.8 mDa. At the same time, using MeteboLynx intelligent software, it can quickly obtain all impurity peaks m/z, retention time, molecular weight, molecular formula, mass deviation, peak area. And the list of impurities of specific structural information, extracting ion flow map, primary and secondary mass spectrum and other information, which greatly saves manpower and time. Through the impurity list and mass spectrum, the possible structure of the impurity can be obtained, and then the impurity secondary structure can be matched by the MassFragment function to determine the impurity structure. From this inference process, it can be seen that with the help of efficient UPLC, accurate QTof instrument and powerful intelligent software, the complex impurity structure identification work becomes simpler and easier, and the personnel experience and qualification background dependence are reduced, which greatly saves manpower. , material and time costs, play a positive role in the research of related substances in drugs. Roundness Measuring Instruments Roundness Measuring Instruments ,Roundness Tester,Roundness Measuring Machine,Instrument Used To Measure Roundness Zhejiang dexun instrument technology co., ltd , https://www.dexunmeasuring.com

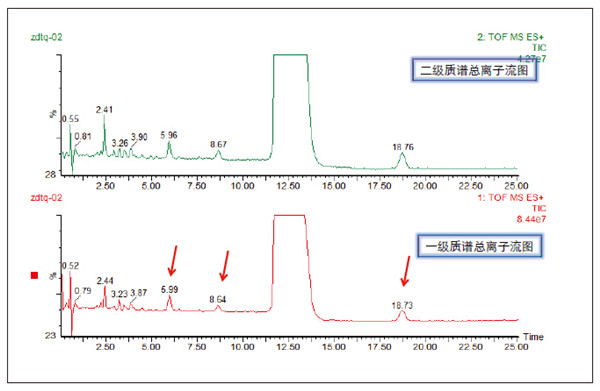
Primary mass spectrometry and secondary mass spectrometry total ion chromatogram