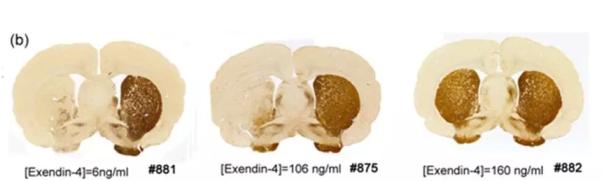
Diabetes drug exenatide has potential to treat Parkinson's disease July 30, 2018 Source: WuXi PharmaTech GLP-1 (glucagon-like peptide-1) receptor agonists are a common class of diabetes drugs. Because of their potential for the central nervous system, scientists are also investigating the possibility of GLP-1 receptor agonists for other neurodegenerative diseases. Recently, researchers at the Korean biotechnology company Peptron have obtained some positive early results and found some neuroprotective effects of exenatide (the main component of Alistin's Byetta and Bydureon). Parkinson's disease (PD) is one of the most common neurodegenerative diseases after Alzheimer's disease, with an incidence of about 1% in people over the age of 60. Although PD has many therapies, none of them have a significant effect on the progression of the disease. Parkinson's disease is mainly caused by the death of dopaminergic (produced dopamine) neurons, and the majority of approved PD drugs, the main mechanism is to enhance the transmission of dopamine signaling. Past studies have shown that damage to the insulin signaling pathway may be associated with the pathogenesis of PD, and epidemiological analysis also suggests a link between diabetes and neurodegenerative diseases. GLP-1 receptors are ubiquitous in the central nervous system and they can play a role in several pathways that enhance cell survival and promote neuroprotection. Therefore, scientists believe that targeting GLP-1 may simultaneously improve glucose metabolism and prevent PD progression. Researchers have previously found that pretreatment with Exendin-4 (the natural hormone mimicked by exenatide) reduces dopaminergic neurodegeneration in animal models, but the use of Exendin-4 in Parkinson's disease is limited by its half-life and blood. Limitation of the brain barrier. The blood-brain barrier prevents certain molecules in the blood from entering the brain, making it difficult to deliver therapeutic doses to the central nervous system. To overcome these shortcomings, Peptron developed a sustained release type exenatide called PT302. In a rat model study of PD, the investigators found that a single dose of PT302 remained high in rat plasma for up to 20 days, while PT302 was higher in cerebrospinal fluid than non-slow release (<6.9 Pg/ml vs. 18.3 to 30 pg/ml). More significantly, in a rat brain damaged by 6-hydroxydopamine, PT302 can significantly increase brain TH-IR (tyrosine hydroxylase immunoreactivity) on the brain injury side, this dopaminergic nerve Meta measurement mark. â–²The results of 3 rats receiving PT302 (Source: "Scientific Reports") This study was recently published by Peptron in the journal Scientific Reports. The current treatment of Parkinson's disease and other brain diseases can only improve symptoms such as improving motor and cognitive function, but can not improve its essential neurodegenerative process. The data in this report show the potential of PT302 to improve the essential neurodegenerative process. . Peptron CEO Dr. Ho-Il Choi said in a statement: "Peer review and publication of these data confirms that Peptron's new SR-exenatide drug crosses the blood-brain barrier and provides long-lasting neuroprotective peptides. An important step in treatment." Peptron is working hard to put PT302 into clinical trials. PD will be the target of choice, and plans to test other diseases such as multiple system atrophy, traumatic brain injury and Alzheimer's disease. We look forward to seeing the clinical results of this drug as soon as possible, opening up new avenues for the treatment of PD. Reference materials: [1] Repurposing a GLP-1 diabetes drug to slow Parkinson's disease [2] Post-treatment with PT302, a long-acting Exendin-4 sustained release formulation, reduces dopaminergic neurodegeneration in a 6-Hydroxydopamine rat model of Parkinson's disease medical Angio Closure Pad,angio syringe,Angio Closure Pad Anesthesia Medical Co., Ltd. , https://www.jssinoanesthesia.com