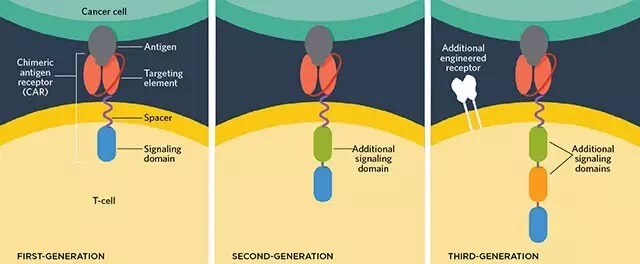
CAR-T cells have produced an unprecedented effect in B-cell malignancies, most notably in the treatment of B-cell acute lymphoblastic leukemia (B-ALL) in CAR-T cells with a complete response rate of 90%. However, the attack of tumor antigens to escape the immune system (cancer immune escape) has become a major challenge for CAR-T cells to treat B-cell malignancies. In addition, the safety issues caused by CAR-T on target/off-tumor in metastatic solid tumors and normal tissues are important obstacles to the success of this treatment. This article reviews some of the mechanisms by which antigen deletions recur and new strategies to address this problem. In addition, the new CAR design is one of the ways we consider enhancing the safety of CAR-T cell therapy in solid tumors. CAR-T cells have produced an unprecedented effect in B-cell malignancies, most notably in the treatment of B-cell acute lymphoblastic leukemia (B-ALL) in CAR-T cells with a complete response rate of 90%. However, the attack of tumor antigens to escape the immune system (cancer immune escape) has become a major challenge for CAR-T cells to treat B-cell malignancies. In addition, the safety issues caused by CAR-T on target/off-tumor in metastatic solid tumors and normal tissues are important obstacles to the success of this treatment. This article reviews some of the mechanisms by which antigen deletions recur and new strategies to address this problem. In addition, the new CAR design is one of the ways we consider enhancing the safety of CAR-T cell therapy in solid tumors. The chimeric antigen receptor (CAR) includes a tumor-associated antigen-binding region (usually derived from the scFV segment of the monoclonal antibody antigen-binding region), an extracellular hinge region, a transmembrane region and an intracellular signal region (CD28, CD137, CD134). T cells that are engineered to express CAR by gene transfer technology are able to specifically recognize their target antigens through the scFv binding domain, resulting in MHC-independent manner of T cell activation. In the past few years, clinical trials in some institutions have demonstrated the efficacy of CAR-T cells targeting CD19, CD20 or CD30 in the treatment of B-cell malignancies including B-cell acute lymphoblastic leukemia (B-ALL), B-cell non-ho Chinchilla lymphoma (B-NHL), chronic lymphocytic leukemia (CLL) and Hodgkin's lymphoma (HL) are effective, and specific CAR-T cells have a response rate of 70-94% for B-ALL. . This remarkable effect will lead to a revolution in the treatment of B-cell malignancies, and opens the door to the application of CAR-T cells for the treatment of solid tumors. Although CAR-T cell therapy has promised a cure for some patients with advanced cancer, there are certain adverse reactions during the treatment. It has been reported that rapid death caused by extra-tumoral cross-reaction of CAR-T cells is Safety is especially important when developing CAR-T cell therapy. Virus Collection And Preservation System Virus Sampling Tube,Covid-19 Virus Collection Kit,Virus Sample Collection Swabs,Virus Collection And Preservation Kit HANGZHOU DIAN BIOTECHNOLOGY CO., LTD. , https://www.dianbiotech.com