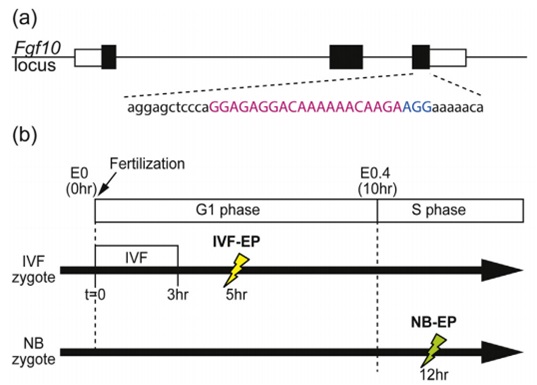
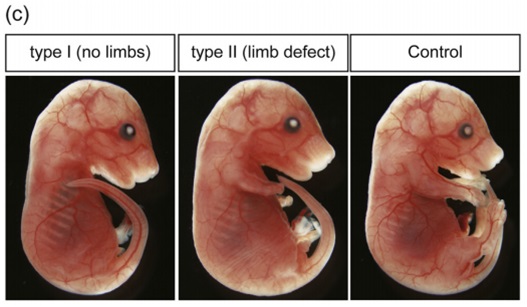
Embryo Gene Editing Analysis: Introducing Cas9 Protein/sgRNA into Mouse Pronuclear Fertilized Eggs to Generate Non-Chimeric Mutants by Electroporation Gene editing technology refers to enabling humans to "edit" a target gene to achieve knockout and addition of specific DNA fragments. Since the advent of CRISPR/Cas9 technology, it has the unparalleled advantages of other gene editing technologies. After continuous improvement, it is considered to be able to "edit" any gene most efficiently and conveniently in living cells. Application direction The following article is a successful explanation of the third application direction. Introduction of Cas9 protein/sgRNA into mouse pronuclear fertilized eggs by electroporation to generate non-chimeric mutants Summary The CRISPR/Cas9 system is widely used in a variety of organisms, especially mice, to elucidate the role of genes. To obtain optimized mouse embryos, Cas9 mRNA and sgRNA are usually introduced into fertilized eggs by injection or electroporation. However, most of the mutants generated by this method are chimeras, containing different types of mutants, resulting in complex phenotypic analysis. In order to simplify gene function analysis, there is an urgent need to find a way to generate non-chimeric mutants. Here, we have identified a method for generating non-chimeric mouse mutant embryos. We simultaneously introduced Cas9 protein and sgRNA into isolated mouse fertilized eggs by electroporation. Make sure that gene editing occurs before the first replication of the mouse genome. As a result, all cells carry a similar mutant phenotype. This approach solves the problem of chimeric mutants produced using the CRISPR/Cas9 system. Materials and Methods Electrotransformation CUY21 EDIT II and LF501 PT1-10 platinum disk electrodes (length: 10 mm, width: 3 mm, height: 0.5 mm, gap: 1 mm) (BEX Co. Ltd., Tokyo, Japan) were used for electrotransformation. The electrodes are connected to the electroconverter and the other end is connected to a stereo Microscope. 30-40 fertilized eggs from mating or in vitro fertilization were simultaneously electrotransfected. The fertilized eggs were cultured in KSOM medium, washed three times with Opti-MEM I, serum was removed, longitudinally aligned in a tank of the electrodes, and 5 uL of Opti-MEM I was added, and Cas9 protein and sgRNA or mcherry mRNA were added. The electrical rotation condition is 7 cycles of 30V (3ms pulse duration + 97ms interval). After electroporation, the fertilized eggs were immediately removed from the shock cell, washed twice with M2 medium and twice with KSOM medium. The fertilized eggs were then cultured in a 37 ° C, 5% CO 2 incubator until a two-cell stage appeared. result The figure generates Fgf10 mutant embryos by electrotransforming Cas9 protein and sgRNA. (a) Fgf10 gene and sgRNA target sequences. (b) The time at which electrical conversion occurs. Electrical rotation occurred 5 hours after in vitro fertilization. Mating fertilization of fertilized eggs occurs at E0.5. (c) Representative embryos exhibit different limb phenotypes: T1 (no limbs) T2 (limbs, no forelimbs or no hind limbs). Control was shocked but did not transfer Cas9 protein and RNA. to sum up We describe this approach by transferring Cas9 protein and sgRNA into in vitro fertilized egg cells to generate mutants. Mutant mice with lower chimerism were generated by NHEJ-mediated deletion insertion or HDR-mediated gene insertion prior to the first replication of DNA. Our electroporation method is very effective in constructing transgenic model mice. Article source Electroporation of Cas9 protein/sgRNA into early pronuclear zygotes generate non-mosaic mutants in the mouse. Developmental Biology. 2016 Autoclave Sterilizer Products,Washer Disinfector,Autoclave Sterilizer,Atomizing Disinfection Robot Guangdong Widinlsa International Co.Ltd , https://www.widinlsa.com