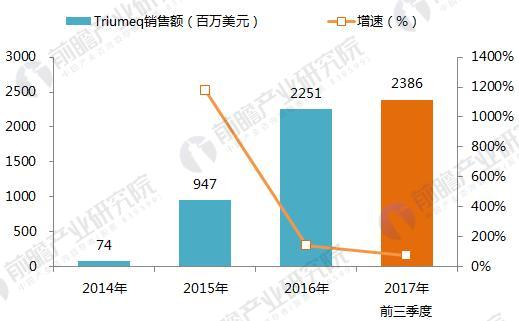
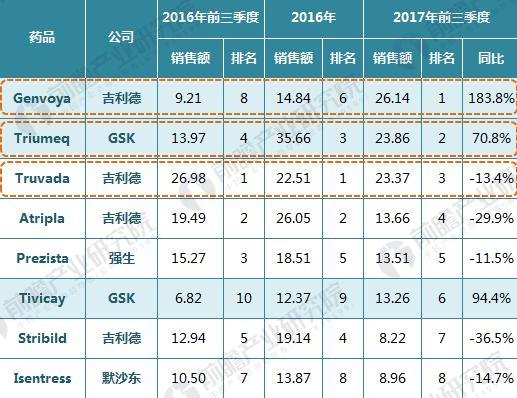
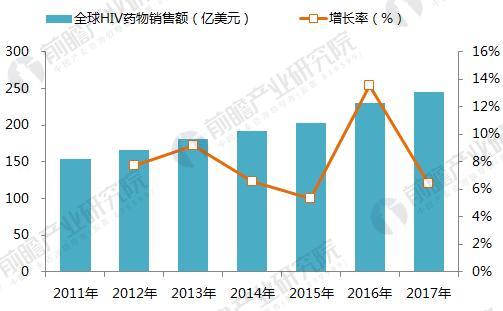
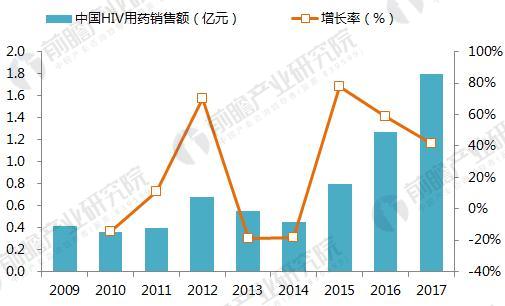
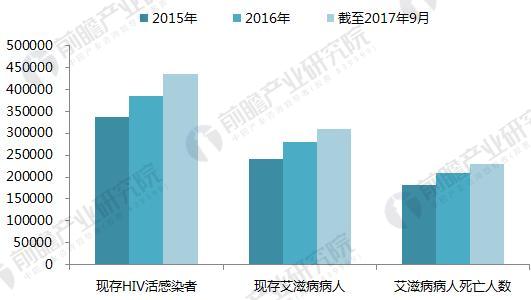
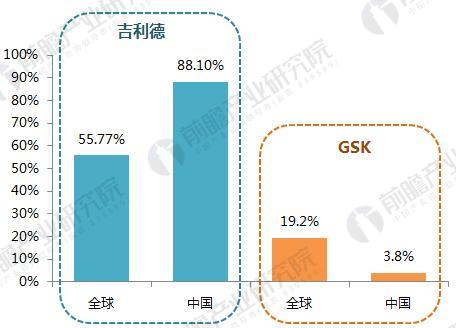
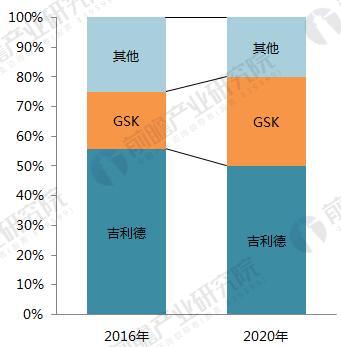
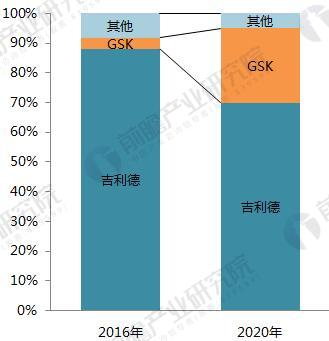
On January 22, 2018, GlaxoSmithKline (GSK) announced its one-piece combination preparation for the treatment of HIV with a new generation of integrase inhibitor, tilivide (DTG). Listed in mainland China. This is the first complete combination of the first complete treatment regimen in the field of HIV treatment in mainland China. Triumeq is a daily 3-in-1 drug developed by ViiV Healthcare, a joint venture between GlaxoSmithKline GSK and Pfizer Pfizer, based on the treatment of the integrase inhibitor Tivicay (Dolutegravir). It also contains two nucleoside reverse transcriptase inhibitors, Abacavir and Lamivudine, which were approved by the FDA on August 27, 2014. Triumeq is highly competitive in the market: on the one hand it combines several different mechanisms of anti-HIV drugs, which has the effect of combination therapy; on the other hand, it is convenient to take, which greatly increases the compliance of patients taking drugs. It is more easily accepted by patients, reducing the impact of patients not being able to take doses on time. Because of its excellent efficacy, the drug entered the ranks of “blockbuster†drugs a year after its launch. In 2015, its sales reached US$949 million, and in 2016 it reached 1.735 billion pounds. $2,251 million), ranked third in all anti-HIV drugs. Triiumeq's global sales for the first three quarters of 2017 reached US$2.386 billion, up 70.8% year-on-year (13.97 in the first three quarters of 2016), more than last year's HIV drug boss Truvada (23 in the first three quarters of 2017) $3.7 billion, and Genvoya, who is about to become the newest anti-HIV drug in 2017, has a gap of less than $300 million. Chart 1: Changes in Triiumeq sales in 2014-2017 (unit: billion US dollars, %) Source: Prospective Industry Research Institute Chart 2: Comparison of sales of anti-HIV drugs in 2017 (unit: billion US dollars) Source: Prospective Industry Research Institute The AIDS epidemic is severe, and the HIV drug market in China is breaking out soon. From the perspective of the global market, anti-HIV drugs are one of the fastest-growing sub-segments. In 2016, global HIV drug sales were US$23.023 billion, and the compound growth rate in 2011-2016 was 8.4%, significantly The average growth rate above the global pharmaceutical market of around 3.5% is also higher than the entire prescription drug market (5.8%, excluding generics). Chart 3: Global HIV drug sales summary for 2011-2017 (unit: billion US dollars, %) Source: Prospective Industry Research Institute Although HIV drugs are mixed in the international market, their development in China is not satisfactory. At present, China's CFDA has approved more than 20 anti-HIV drugs, but few patients in the hospital are treated, 2016. In the year, the overall size of China's anti-HIV drug market (calculated according to the retail price of the terminal) was about 127 million yuan, which is simply a comparison with the global market. Chart 4: Summary of sales of HIV medications in China's sample hospitals in 2009-2017 (unit: 100 million yuan, %) Source: Prospective Industry Research Institute However, from the perspective of demand, the number of people living with HIV in China has grown rapidly. As of September 2017, there were 746,644 cases of HIV-infected and AIDS patients (2,278,78 cases) and 436,587 cases of AIDS-infected people. 310057 cases of AIDS patients. The huge HIV patient population has strong support for HIV drug demand. About 60% of people currently receive antiretroviral treatment, but there is still a gap in achieving the “three 90%†goal by 2020. The new drugs of foreign pharmaceutical giants have been approved in China, and the HIV drug market in China will usher in an outbreak. Figure 5: The situation of AIDS and HIV-infected people in China is very serious Source: Prospective Industry Research Institute Triumeq is expected to help GSK compete with Gilead in the anti-HIV market At present, the global anti-AIDS drugs are concentrated in Girard, GSK, Johnson & Johnson, Merck and other four European and American multinational pharmaceutical companies. In 2016, only Gilead's HIV drug sales reached US$12.84 billion, accounting for 55.8% of the world is the “big brother†who is well-deserved in the anti-AIDS market; the second and third ranked GlaxoSmithKline (GSK) and Bristol-Myers Squibb (BMS) accounted for 19.2%, respectively. 8.6%, the gap with Gilead is large. Domestic HIV drugs are more concentrated than the global market, with the Gilead family accounting for 88% of the market, and GlaxoSmithKline's share is only 3.8%. Figure 6: Comparison of global and domestic anti-HIV drug market share in 2016: Gilead and GSK Source: Prospective Industry Research Institute However, compared with several other anti-HIV star drugs, Triumeq is one of the most promising drugs. Although the 2017 anti-HIV drug champion is expected to be won by “Genvoyaâ€, the difference between Triumeq is very small. (The gap in the first three quarters of 2017 was less than $300 million.) After defeating Truvada, it is expected to lead GSK to compete with Gilead in the anti-HIV market. Figure 7: Global 2020 HIV drug competition pattern forecast (unit: %) Source: Prospective Industry Research Institute Figure 8: Forecast of China's HIV drug competition pattern in 2020 (unit: %) Source: Prospective Industry Research Institute Flounder Flounder,Flounder Def,Frozen Flounder,Cooking Frozen Flounder ZHEJIANG EVERNEW SEAFOOD CO.,LTD , https://www.evernewseafood.com