Abstract: Non-PVC composite film infusion bag has the advantages of eliminating bacterial environment, convenient use, high gas barrier and water resistance, and environmental protection. It is widely used as an infusion packaging material. However, such material infusion bags often encounter cracking problems during transportation and storage, and the causes of product damage are mostly related to the material's own tensile properties or heat sealing effect. Therefore, reasonable testing instruments and test methods are used to monitor the tensile strength and bursting pressure of the infusion bag to achieve effective screening. The infusion bag rupture is caused by the deterioration of the mechanical strength of the material or the rupture of the edge banding. This paper adopts XLW (PC) intelligent electronic tensile testing machine and LSSD-01 leakage and sealing strength tester independently developed by Jinan Languang Electromechanical Technology Co., Ltd., and introduces specific monitoring schemes for relevant industries with reference to relevant inspection standards. Key words: non-PVC composite film, infusion bag, mechanical strength, tensile strength, tensile strength, tensile properties, heat sealing effect, edge cracking, undercutting, excessive heat sealing, poor heat sealing, bursting pressure, fracture location , heat seal strength, leak and seal strength tester, intelligent electronic tensile tester, pharmaceutical, soft plastic packaging, pharmaceutical packaging 1 , detection significance The infusion method has changed from the original open development of Zui to the now fully enclosed process. The infusion set has also been transformed from the original glass bottle into a simple polyvinyl chloride (PVC) soft plastic infusion bag. The material of the infusion bag is also non-environmentally friendly. The PVC material is upgraded to a non-PVC composite film pouch that is fully environmentally friendly. The material quality of non-PVC composite film infusion bag meets the standards of European, Japanese and American multi-national pharmacopoeia. It has high moisture resistance and oxygen barrier properties and is suitable for the packaging of most liquid drugs. The infusion bag of this material is made of polypropylene (PP), polyethylene (PE), polyamide (PA) and a variety of elastic materials (SEBS) in a co-extrusion process produced by a co-extrusion process under 100 clean conditions. Because it does not contain plasticizer and uses a co-extrusion process, it only produces water and carbon dioxide oxides after being incinerated, and is environmentally friendly. Therefore, non-PVC composite film has become the main infusion bag material. The non-PVC composite film infusion bag adopts materials with higher density of PP and PA in the middle layer and outer layer. It can effectively improve the mechanical strength of the infusion bag based on the enhanced water vapor and oxygen barrier properties of the infusion bag, and is not easy to be broken and easy to transport. And storage. However, the ratio of PP, PE, PA in the infusion bag of such materials is not suitable or the production process is improperly operated, which may cause the mechanical strength to decrease, that is, the tensile strength and the tensile deformation rate of the bag material are lowered, and the bag body is easily broken; If the infusion bag is in the process of heat sealing of the sealing edge, the infusion bag is also easy to be caused by the "dummy seal" caused by the heat seal strength being too low or the "root cut" or "root breakage" caused by excessive heat seal. Cracking at the edge of the seal during transportation and storage. Therefore, the monitoring of mechanical properties such as toughness and bag compression resistance of non-PVC composite film infusion bags is essential. This paper starts from the testing of tensile strength and bursting pressure, and combines XLW (PC) intelligent electronic tensile testing machine and LSSD-01 leakage and sealing strength tester independently developed by Jinan Languang Electromechanical Technology Co., Ltd. The reason for the rupture of PVC composite membrane infusion bag and its monitoring program provide reference for the pharmaceutical industry. Figure 1 Non-PVC composite film infusion bag 2 , testing standards At present, the domestic testing of tensile strength of infusion bags is based on YBB00112003 "Tensile Performance Measurement Method", which is used for the inspection of tensile properties of pharmaceutical packaging. For the test of burst pressure of infusion bags, there is currently no clear standard for testing methods in China. In this paper, ASTM F1140 “Test Method for Resistance to Internal Pressure Damage of Unconstrained Packaging Materials†is used to test the burst pressure of the infusion bag, verifying the position where the infusion bag is prone to rupture, and combining the above tensile strength test results to determine that the rupture phenomenon is caused by the infusion bag The poor stretch performance is also caused by the tight sealing of the seal or the excessive heat seal. 3 , test samples A brand of glucose infusion bag (non-PVC composite film material). 4 , testing equipment This article covers two testing equipments, namely XLW (PC) intelligent electronic tensile testing machine and LSSD-01 leakage and sealing strength tester. Both testing equipments are independently developed and produced by Jinan Languang Electromechanical Technology Co., Ltd. Figure 2 XLW (PC) intelligent electronic tensile testing machine Figure 3 LSSD-01 leakage and seal strength tester 4.1 Principle of the test (1) XLW (PC) intelligent electronic tensile testing machine The sample is clamped between the two chucks of the clamp, and the two chucks are moved relative to each other. The force value sensor and the built-in displacement sensor on the movable chuck are used to collect the force value change and displacement during the test. Change to calculate various mechanical properties of the sample. (2) LSSD-01 leakage and seal strength tester Using the principle of positive pressure method, the instrument is used to pressurize the inside of the package to be tested until the package is broken. Pneumatic pressurization and pressurization devices have the ability to continuously pressurize to maintain a gradual increase in pressure until the package breaks. This method detects the large pressure of the package before the package breaks and determines where the package is easily broken. 4.2 Scope of application (1) XLW (PC) intelligent electronic tensile testing machine (2) LSSD-01 leakage and seal strength tester 4.3 Equipment parameters (1) XLW (PC) intelligent electronic tensile testing machine (2) LSSD-01 leakage and seal strength tester 5 , the test process 5.1 Test procedure for tensile strength index 5.2 Test procedure of burst pressure index (1) Place the infusion bag sample in an environment of 23 ± 2 ° C and 50 ± 5% RH for 48 hours or more. (2) For the sealed infusion bag sample, take a probe to puncture the bag body and pressurize the inside of the bag. (3) Select the “Fracture Test†method in the ASTM F1140 standard, place the fully sealed infusion bag on the LSSD-01 Leak and Seal Strength Tester , and carefully insert the pressurizing probe into the center of the bag . (4) Record the position of the insertion point and place all the probe insertion holes of the test sample in the same position. (5) When inserting the probe, avoid puncturing other faces of the infusion bag. If the packaging material is easily torn, tape or other equivalent means should be used to enhance the mechanical strength of the material at the insertion point and its vicinity. (6) Click the start button, the test starts, and the bag is inflated to expand. Continue to pressurize until the bag breaks. (7) Check the infusion bag after the test, and record the position, type, and pressure value at the time of rupture (bag or seal). Figure 4 Blasting pressure test process 6 , test results The bursting pressures of the three samples of the non-PVC composite film infusion bag tested in this paper were 80.6 KPa, 71.1 KPa, and 72.4 KPa, respectively, and all three samples were broken at the root position of the right edge of the infusion bag. The average longitudinal and transverse tensile strengths of the five samples of the infusion bag were 35.77 MPa and 28.40 MPa, respectively. Therefore, from the tensile strength results, the tensile performance of the infusion bag sample is better. Combined with the results of the burst pressure test, it can be determined that the position of the infusion bag is easily broken mainly at the root of the right edge, indicating that the right side of the infusion bag is overheated. It is easy to cause the root to break. 7 , conclusion The rupture of the non-PVC composite film infusion bag is a headache for the quality of the product during transportation and storage. The XLW (PC) intelligent electronic tensile testing machine and the LSSD-01 leakage and sealing strength tester can be used for comprehensive testing. Verify the location of the infusion bag that is easily broken and explore the cause to improve the breakage rate. Labthink is committed to providing professional testing equipment and services to customers around the world. In addition to the above two testing equipments, Labthink can also provide you with medical packaging related to barrier properties, various physical and mechanical properties, and hygienic performance. Testing equipment, detailed information about the equipment can be found at Languang or directly by telephone. The more you understand, the more you trust! Labthink is looking forward to enhancing technical exchanges and cooperation with enterprises and institutions in the industry! Copyright statement: Article Copyright Jinan Languang Electromechanical Technology Co., Ltd., reprinted without permission!
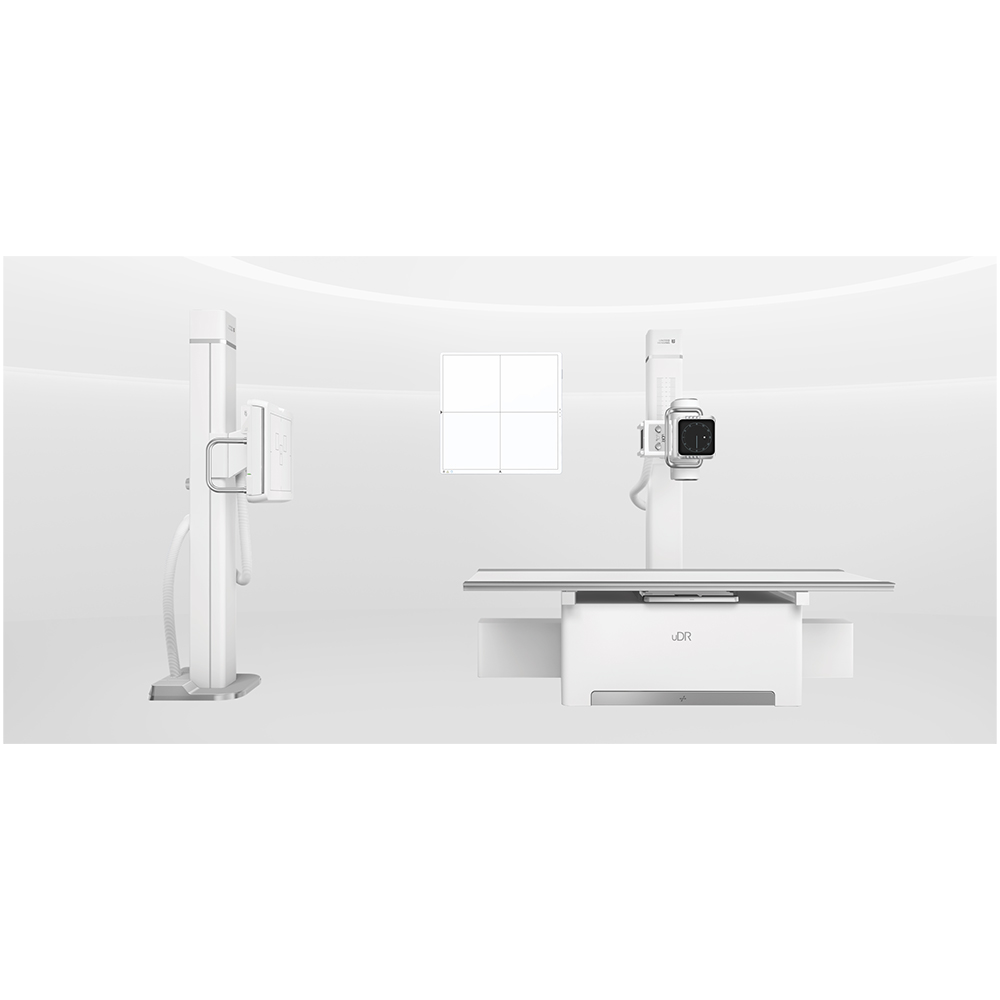
Digital Radiography made affordable, Created to meet the needs of community to hospitals and private radiology practices, it enables the price-sensitive customers to join the drive to go digital.The versatile floor mounted radiography system enables you to go from film to CR to DR. Flooe-mounted easy for installation and operation.
United-imaging according to the international advanced processing mode and standardlize design. the parts are made with precision CNC machine and moulding. Which adopt high degree of standardized, reasonable and compact structure. With reliable, durable and elegant appearance and advanced processing technology.
X-Ray Digital Machine,X-Ray Digital Machine In Medical,X-Rays Digital Medical Machine,Medical X-Ray Digital Imaging Machine Shanghai Rocatti Biotechnology Co.,Ltd , https://www.ljdmedical.com



The United-imaging is floor mounted system comprises a radiographic table with integral floor guide rail and a wallstabd. Requiring little room preparation, it is easy to install.