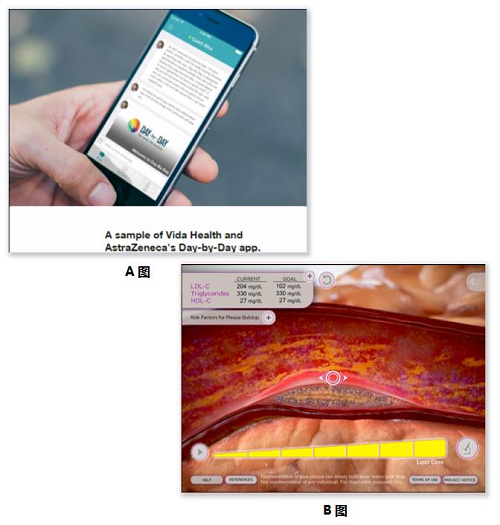
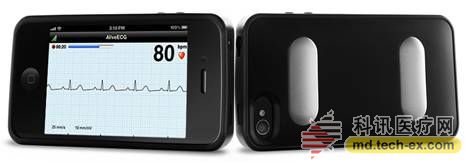
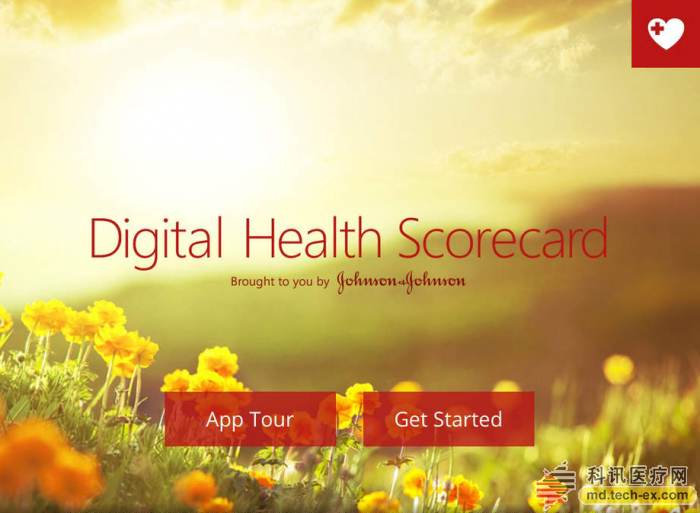
Release date: 2015-08-20 Last October, a research report by Research2Guidance showed that although the pharmaceutical giant developed a lot of apps, the download volume was not satisfactory. The survey sample was taken from 725 APPs from 11 world-renowned pharmaceutical companies (see photo below). In August of this year, the latest survey from German APP company SmartPatient found that 66% of the apps developed by pharmaceutical companies were distributed in the developed countries of the United States, the United Kingdom and Germany. It can be seen that the general public on a global scale still feels unfamiliar with Internet medical care and is far from forming a habit. In the cardiovascular field, overseas famous pharmaceutical companies are still taking the lead in the development of intelligent hardware, wearable devices and APP, while the traditional traditional pharmaceutical companies in China have not performed any performance. Merk On August 22, 2014, Merk acquired eCardio. eCardio can provide a variety of ECG monitoring equipment to help doctors improve the diagnosis of arrhythmia and other symptoms. Its remote diagnostics service is also used on heart rate monitoring devices that connect Alivecor to smartphones. On September 9, 2014, eCardio merged with wearables and remote monitoring equipment manufacturer Preventice, and Merk Global Health Innovation Fund (Merk GHI) funded the development of Preventice's BodyGuardian heart rate sensor. On October 7, 2013, Merk GHI acquired Omnio, an Internet health company. The acquisition means acquiring Omnio's iPad app, a tool that provides medical personnel with quick and easy access to medical information. It contains medical guidance for heart disease atrial fibrillation. Astrazeneca On July 14, 2015, Astrazeneca and Vida Health wanted to jointly develop an APP that would help patients with a first heart attack recover. The APP is named Day-By-Day, which complies with the HIPAA confidentiality agreement and is first tested at Duke University to help patients change their lifestyle and take medication according to digital education guidelines such as videos, articles, dietary advice, etc. The first attack occurred in a second heart attack. It is also a content extension of Vida Health's existing mobile app about chronic disease self-health management. Astrazeneca has also developed medical education tools that allow doctors to show patients the shape of atherosclerosis in an animated form, facilitating patient-to-patient discussion. The patient's LDL, HDL, and triglyceride values ​​can be entered and the snapshot image of any stage can be emailed to the patient via the reporting function. Pfizer On May 24, 2012, Pfizer and EatingWell magazine collaborated to develop an app for hyperlipidemic patients. The APP is only for patients taking the lipid-lowering drug Lipitor, including EatWell magazine's shopping list tool, regular cooking tools and recipe query tools, and Lipitor's $4 co-payment card. But the good times are not long, this app will soon be removed. Pfizer also develops an app for a healthy little secretary. It is a health gadget that records all the indicators. It can record BMI, weight, blood pressure, heart rate, blood sugar, allergy records, etc. It can also set medication reminders and hospital searches. , common disease inquiries, etc. Boehringer Ingelheim On January 19, 2011, Boehringer Ingelheim developed an intuitive and easy-to-use app called NIHSS (National Institutes of Health Stroke Scale (USA)) to help doctors perform NIHSS ratings for stroke patients. Now this app has also been removed. Boehringer Ingelheim has also developed a tool for medical professionals to develop risk information for non-valvular atrial fibrillation strokes, including a stroke risk calculator, as well as a bleeding risk calculator and renal function calculation and management tools. Some of the results can be emailed to the patient. Another APP, developed for professional stroke personnel (HCP), calculates the disease grade of patients with acute ischemic stroke and related counseling information, including NIHSS calculations, taking antithrombotic Actilyse doses, and managing patient health. Pfizer, Boehringer Ingelheim, Bayer On August 20, 2013, iECG, developed by AliveCor, a US company supported by Khosla Ventures, officially entered the hospital and received medical tests from doctors. This heart monitor was previously called the iPhone ECG, which captures abnormal ECG patterns from patients. University of Sydney professor Ben Freedman published a research report on the accuracy of iECG at a heart conference, resulting in an accuracy rate of 97%. Ben Freedman's research was funded by Pfizer, Boehringer Ingelheim and Bayer, but the inventor of iECG was not Ben Freedman, but American heart expert Dr. Dave Albert and two Australians Bruce Satchwell and Kim Barnett. Sanofi Aventis On January 19, 2011, Sanofi Aventis developed a free app called AFib Educator to help doctors explain AFib (heart atrial fibrillation) to patients, their families or caregivers. It interactively demonstrates how atrial fibrillation affects normal heart function, including disease symptoms, risks, and management strategies, with detailed animations and color pictures that distinguish between normal and atrial fibrillation. And X-ray fluoroscopy images in the case of cardiac arrhythmias; there are also contrasting electrocardiograms of normal and abnormal hearts. Novartis On August 13, 2014, Seventh Sense announced that it has received $16 million in Series B investment from three major companies, Novarti, Siemens and Burlington CNC Labs. It is used to further develop the blood collection device TAP for inspection and diagnosis. This small, portable device has a stethoscope-head size that pierces the skin with a hydrogel and draws a little force to get the patient's blood. Generally, blood samples can be taken from 20 to 100 microliters, and then the patient's blood is extracted by means of siphoning and vacuum pressure. The biggest advantage is that the patient does not feel pain, only feels vacuum suction, and can not see the blood, to avoid blooming (above). On September 22, 2009, Novartis and Proteus Biomedical, an innovative company in the development of electronic medicine, conducted a drug-taking experiment to observe patients with hypertension. The drug was Diovan and tracked the operation of the drug chip in these 20 subjects. In the case, the signal can be reflected on the receiving sensor worn on the patient's shoulder. The results showed that patient medication compliance increased from 30% to 80% during the six months. On November 11, 2010, Novartis wanted to load Proteus Biomedical's invention patent IEM, microchip technology into tablets. After the patient took it, the stomach acid in the stomach activates the chip, thereby transmitting heart rate, body temperature and body movement. Go to the skin patch of the Bluetooth connection. The skin patch then transfers the data to the EMR, allowing the doctor to view the patient's body data remotely. On July 10, 2015, Novartis' new cardiovascular disease drug Entresto was approved by the FDA. CEO Joe Jimenez said that although the price of new drugs is relatively high, it costs an average of $12.5 per day. However, if Internet devices are used to remotely monitor medication and improve patient compliance, it will save money in the long run. On November 19, 2013, the Avatar team won the Novartis Internet Medical Challenge Award. The contest centered on the theme of heart failure, a total of 200 companies and developers came to compete for the competition for $40,000. Developed by Sense.ly, this app helps doctors remotely monitor patients with congestive heart failure, with speech recognition, capture of qualitative and quantitative data, and physician advice. Novartis, Roche On December 2, 2010, Fujifilm Japan launched a waterproof mobile phone F-02C, becoming the first mobile phone to receive the Continua Health Alliance certification, which can be connected to measurement equipment. An app called i-appli on the phone measures blood pressure and body composition. Continuas is a medical health organization of 236 corporate members dedicated to the interoperability of medical devices. Continua members include large technology companies IBM, Cisco, Microsoft, and pharmaceutical companies Novartis, Roche and health insurance companies St. Jude Medical, Ascension Health, and Others. GlaxoSmithKline(GSK) On November 17, 2014, GSK teamed up with emerging data company Medidata and sensor manufacturers Vital Connect and ActiGraph to conduct small experimental studies using mobile devices to evaluate the feasibility of wearable sensors in clinical diagnostics. The study involved only six topics, of which the Human Performance Laboratory tracked and measured a series of vital signs, including ECG data and active performance, for six healthy participants. Johnson & Johnson Johnson & Johnson's Janssen Healthcare Innovations provides patients with a mobile medical solution called Care4Today. Care4Today has four major programs, one of which is the heart health field. Its core components are: 1. Customized technology platform, combined with the hospital's existing IT system, providing an intuitive technology platform for the convenience of hospital staff; 2. Management of medical staff and patients Both sides, multimedia mode of behavior education and incentives for patients, change patient habits, support the cardiac rehabilitation team to provide medical services to patients; 3. Implantation experiments, strict project implementation programs, training and business development for medical service personnel. J&J has developed a tracking health app, Track Your-Health, which displays data on a variety of health devices, including daily activity, calorie intake, custom personal goals, as well as high-density lipoprotein and low-density lipoprotein in the body. Content test. J&J has also developed a digital medical scoring tool APP that allows users to self-assess the risk of chronic diseases such as diabetes, cardiovascular disease, respiratory disease or cancer. Take a few minutes to answer questions about your health and lifestyle habits, get a health score, and get BMI, cholesterol, blood sugar and more. Abbott On April 4, 2011, the FDA approved Abbott's i-STAT 1 wireless blood analyzer. This instrument can help medical personnel share or deliver critical medical information without having to accompany the patient bed every day. For example, the patient underwent a cardiac troponin assay kit, and the emergency room was able to measure, transmit, and treat patients under remote guidance from an American Heart Association specialist in a different location to support the final diagnosis of heart disease. Abbott has also developed tools for women's cardiovascular health, helping medical workers educate patients about heart health prevention. The educational function is embodied in three aspects. One is to learn the characteristics of the disease and the risk factors. The second is to let the women self-test and self-test in the form of games. The third is to connect directly with ForYourHeart.com, an Abbott self-built heart education website (Figure 1). There is also an APP, a patient-oriented video education app that US medical staff can use to demonstrate cardiovascular health and interventional procedures to patients. These include drug release stent video display, carotid stent video display, coronary artery stenting surgery, peripheral vascular disease, and stent surgery (Figure 2). Another app, also a patient education tool, uses realistic enhancements to show the patient the procedure (Figure 3). Merck Sharp & Dohme (MSD) MSD developed a calculation tool for Italian medical staff to calculate cholesterol, low-density lipoprotein, and cardiovascular risk. Daichii Sankyo On March 3, 2015, Boston's Partners HealthCare and Japanese pharmaceutical company Daichii Sankyo announced a collaboration on anticoagulant drugs for patients with atrial fibrillation. Because high-risk atrial fibrillation patients need anticoagulant therapy, the project is named “mobile wrap†-around". In the future, we will develop wearable testing equipment and supporting APP to help patients receive timely advice and responses from doctors. Source: Arterial Network Antimicrobial Hemodialysis Catheter Hemodialysis Catheter,dialysis Catheter,powertrialysis dialysis catheter,peritoneal dialysis catheter,dialysis catheter kit Anesthesia Medical Co., Ltd. , https://www.jssinoanesthesias.com